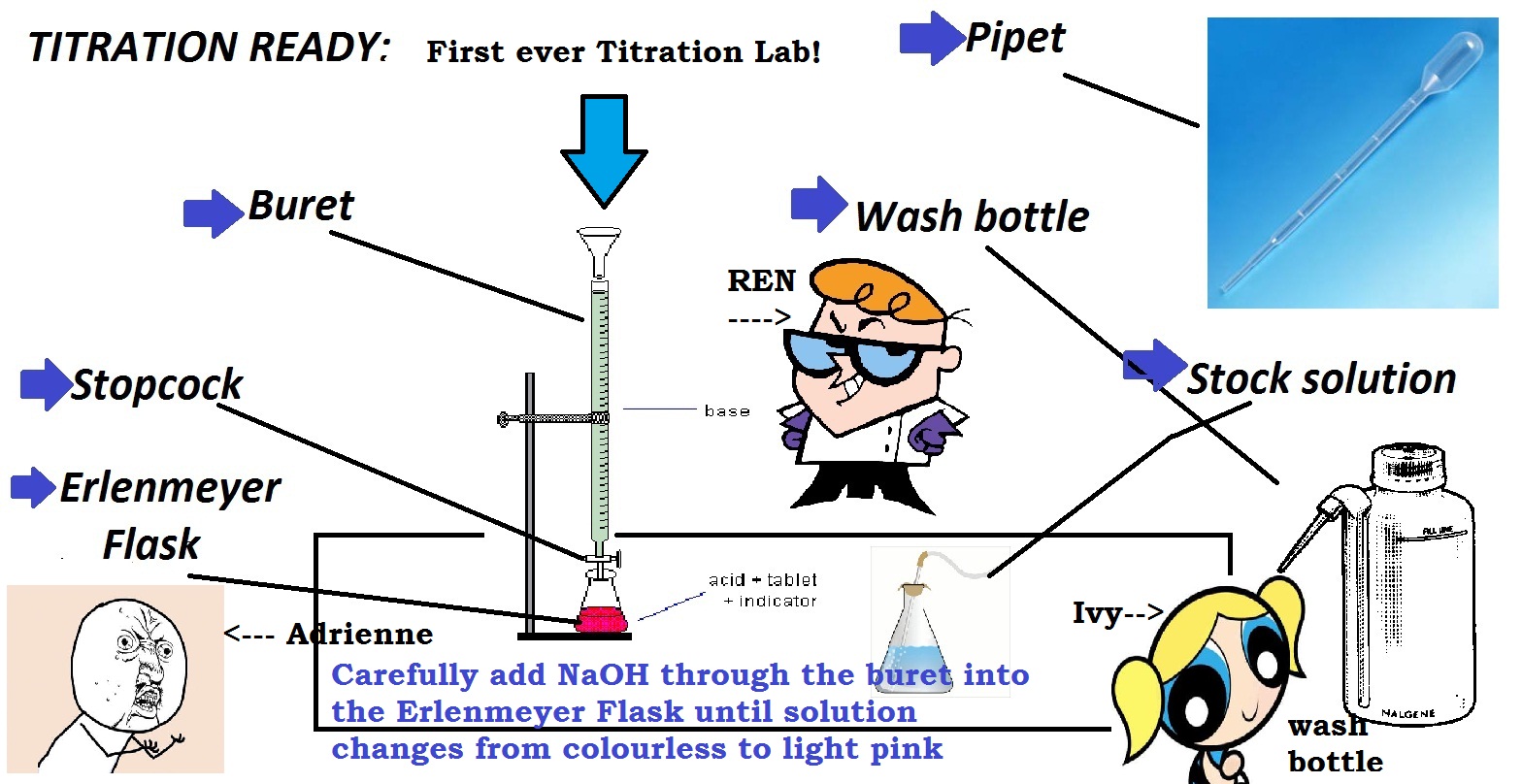
Welcome to our kitchen table titration lab! In this article, we will be exploring the world of chemistry through a hands-on experiment that you can easily do in the comfort of your own home. Not only is this a fun and interactive way to learn about titrations, but it also allows you to appreciate the science behind everyday household items. So put on your lab coat and let's get started!Introduction
The first step in any experiment is gathering all the necessary materials. For this titration lab, you will need:Gathering Materials
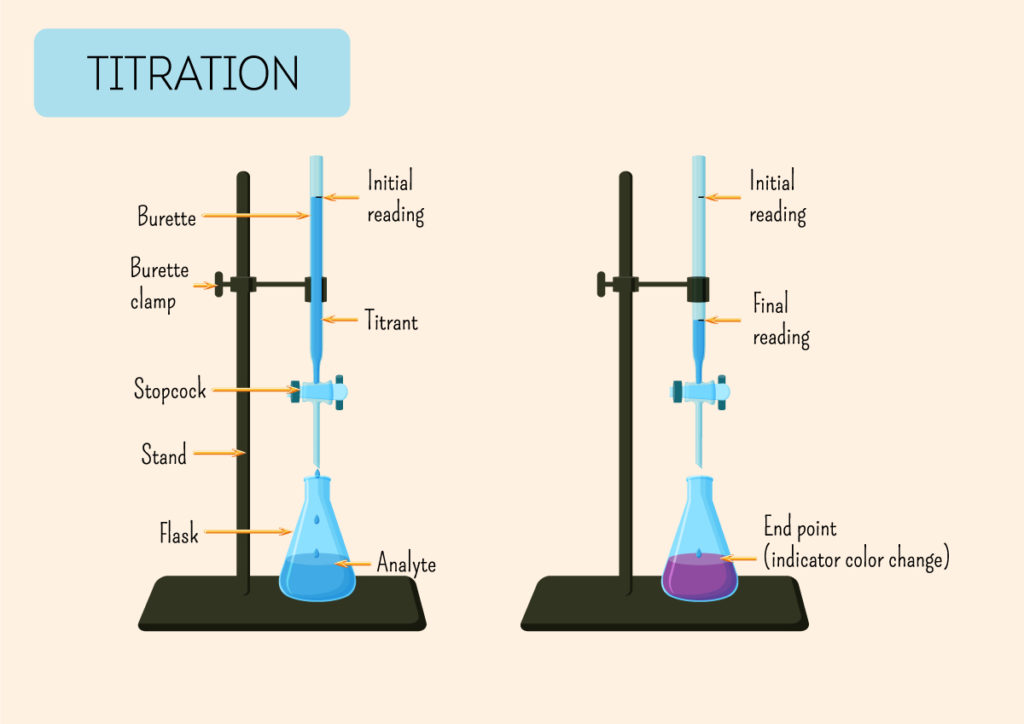
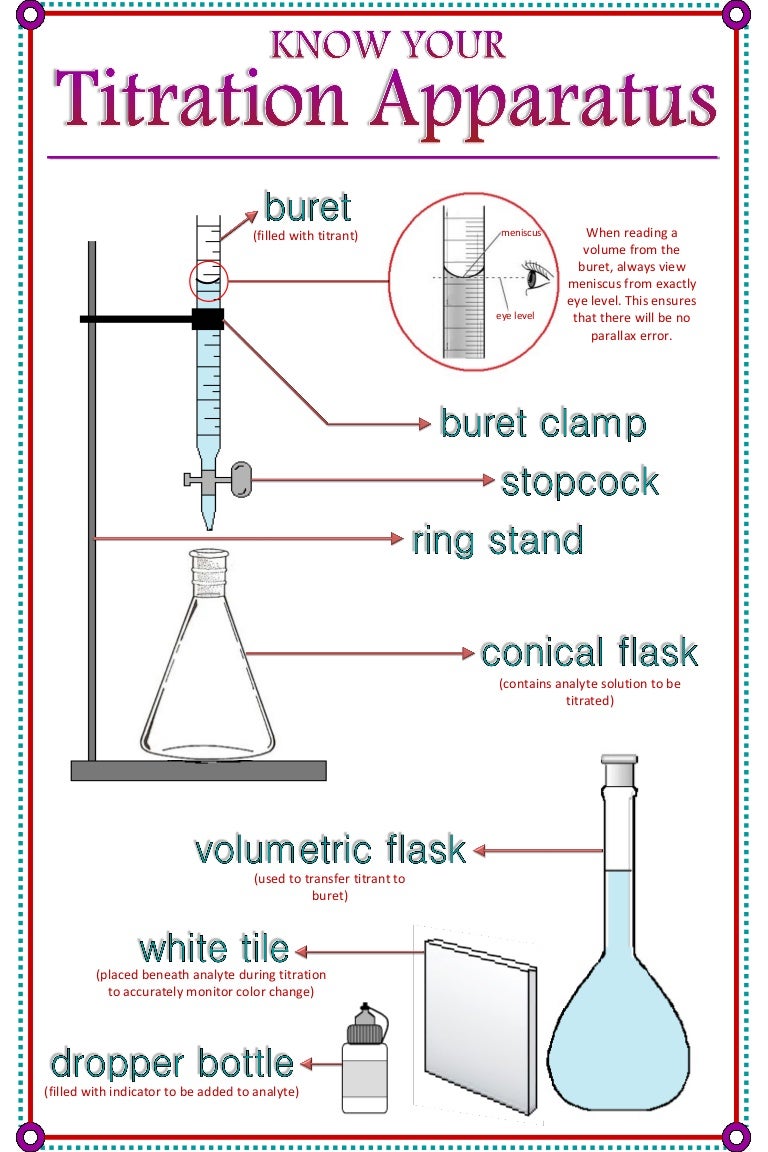
Now that you have all your materials, it's time to set up your titration lab. Start by filling the burette with your basic solution and placing it on the stand. Use the clamp to secure it in place. Next, fill your volumetric flask or graduated cylinder with your acidic solution and add a few drops of your chosen indicator. The indicator will change color when the titration is complete, so choose one that best suits your experiment.Setting Up
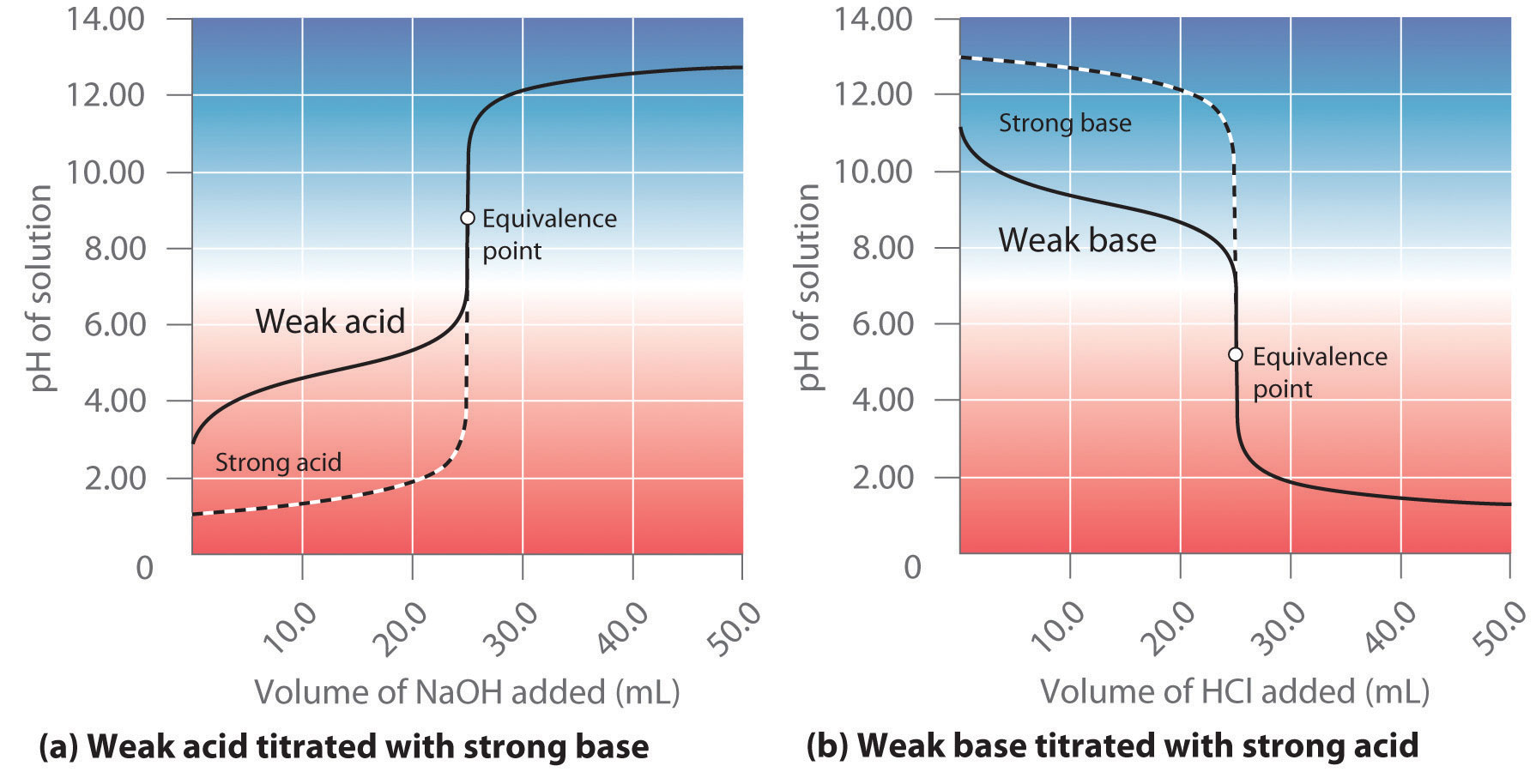
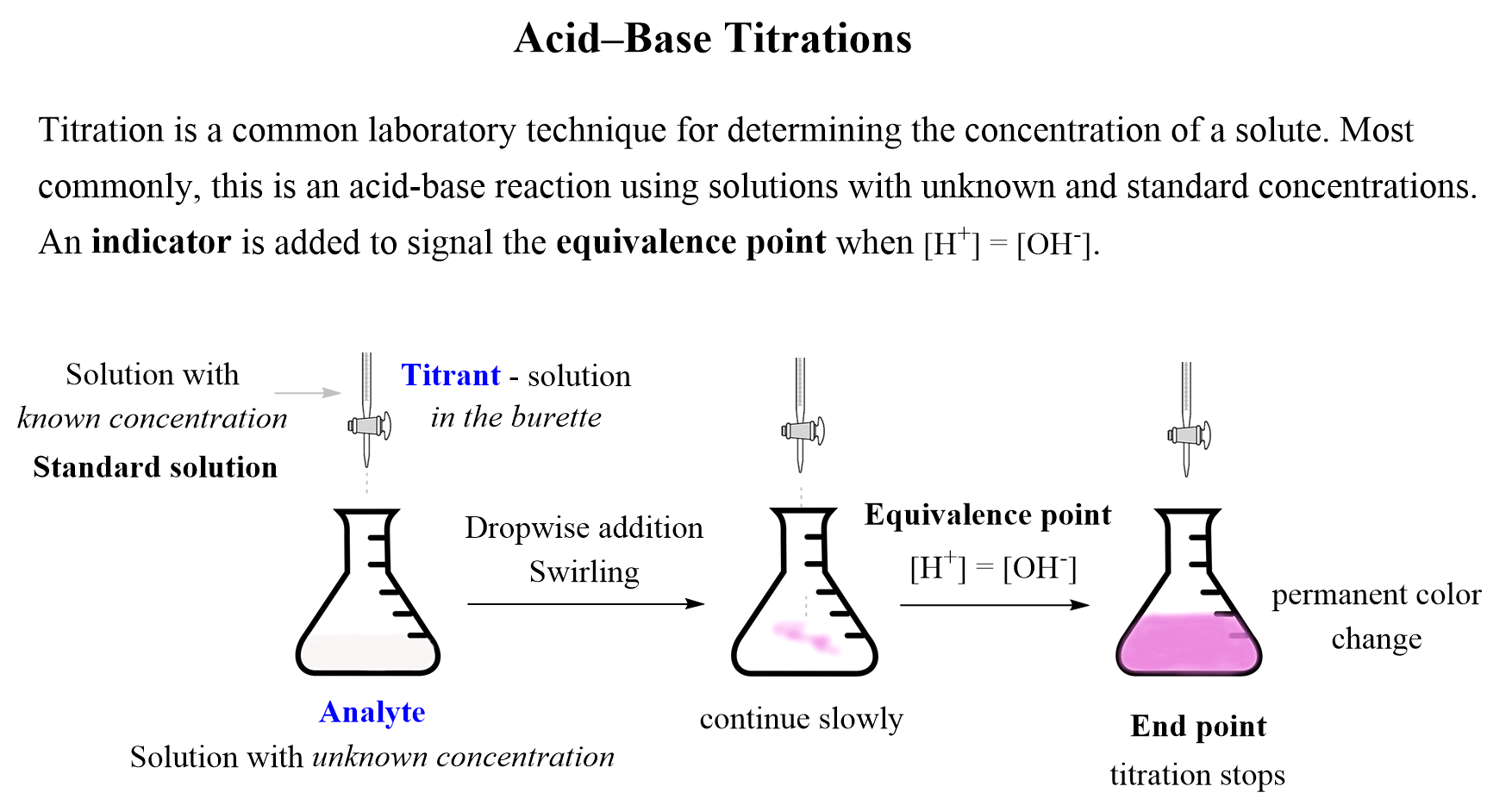
It's time to start the titration! Use the burette to add the basic solution to the acidic solution, one drop at a time. Swirl the flask or cylinder after each drop to ensure the solutions are mixing properly. As you approach the endpoint, the color of the indicator will change. This indicates that the reaction is complete and you have reached the equivalence point.Performing the Titration
Now that the titration is complete, it's time to calculate the results. Use the volume of your basic solution and the concentration of your acidic solution to determine the amount of acid in the solution. This calculation is known as the titration equation and can be found in any chemistry textbook. Remember to record your results and any observations you made during the experiment.Calculating the Results
Now that you have mastered the basic titration, why not try exploring further? You can change the concentrations of your solutions or try using different indicators to see how they affect the results. You can also test different household items to see if they are acidic or basic. This is a great way to apply your newly learned skills and expand your knowledge of chemistry.Exploring Further
Titration is not just a fun experiment, but it also has many real-world applications. It is commonly used in the medical field to determine the acidity or alkalinity of a patient's blood. It is also used in the production of various products, such as food and pharmaceuticals, to ensure the correct amount of acid or base is present. By learning about titration, you are gaining a better understanding of the world around you.Real-World Applications
Congratulations, you have successfully completed your kitchen table titration lab! You have learned about the importance of safety and accuracy in experiments, as well as how to perform a titration and calculate the results. We hope you had fun and gained a newfound appreciation for chemistry. Now, go clean up your lab equipment and don't forget to wash your hands!Conclusion
<h2>Introduction</h2> <p>Welcome to our kitchen table titration lab! In this article, we will be exploring the world of chemistry through a hands-on experiment that you can easily do in the comfort of your own home. Not only is this a fun and interactive way to learn about titrations, but it also allows you to appreciate the science behind everyday household items. So put on your lab coat and let's get started!</p> <h2>Gathering Materials</h2> <p>The first step in any experiment is gathering all the necessary materials. For this titration lab, you will need:</p> <ul> <li><i>Acidic solution (such as vinegar or lemon juice)</i></li> <li><i>Basic solution (such as baking soda or ammonia)</i></li> <li><i>Distilled water</i></li> <li><i>Measuring cups and spoons</i></li> <li><i>Graduated cylinder or volumetric flask</i></li> <li><i>Titration setup (burette, stand, and clamp)</i></li> <li><i>Indicator solution (such as phenolphthalein or litmus)</i></li> </ul> <p>Make sure to read the instructions on the label of each solution and handle them with caution. Safety first!</p> <h2>Setting Up</h2> <p>Now that you have all your materials, it's time to set up your titration lab. Start by filling the burette with your basic solution and placing it on the stand. Use the clamp to secure it in place. Next, fill your volumetric flask or graduated cylinder with your acidic solution and add a few drops of your chosen indicator. The indicator will change color when the titration is complete, so choose one that best suits your experiment.</p> <h2>Performing the Titration</h2> <p>It's time to start the titration! Use the burette to add the basic solution to the acidic solution, one drop at a time. Swirl the flask or cylinder after each drop to ensure the solutions are mixing properly. As you approach the endpoint, the color of the indicator will change. This indicates that the reaction is complete and you have reached the equivalence point.</p> <h2>Calculating the Results</h2> <p>Now that the titration is complete, it's time to calculate the results. Use the volume of your basic solution and the concentration of your acidic solution to determine the amount of acid in the solution. This calculation is known as the titration equation and can be found in any chemistry textbook. Remember to record your results and any observations you made during the experiment.</p> <h2>Exploring Further</h2> <p>Now that you have mastered the basic titration, why not try exploring further? You can change the concentrations of your solutions or try using different indicators to see how they affect the results. You can also test different household items to see if they are acidic or basic. This is a great way to apply your newly learned skills and expand your knowledge of chemistry.</p> <h2>Real-World Applications</h2> <p>Titration is not just a fun experiment, but it also has many real-world applications. It is commonly used in the medical field to determine the acidity or alkalinity of a patient's blood. It is also used in the production of various products, such as food and pharmaceuticals, to ensure the correct amount of acid or base is present. By learning about titration, you are gaining a better understanding of the world around you.</p> <h2>Conclusion</h2> <p>Congratulations, you have successfully completed your kitchen table titration lab! You have learned about the importance of safety and accuracy in experiments, as well as how to perform a titration and calculate the results. We hope you had fun and gained a newfound appreciation for chemistry. Now, go clean up your lab equipment and don't forget to wash your hands!</p>HTML Code
The Importance of Kitchen Table Titration Lab for House Design

Creating a Functional and Aesthetically Pleasing Kitchen
 The kitchen is often considered the heart of the home, where families gather to cook, eat, and share stories. As such, it is crucial to design a kitchen that is not only functional but also visually appealing. This is where a kitchen table titration lab comes in.
Kitchen table titration lab
is a process where homeowners can experiment with different design elements and materials for their kitchen in a controlled and cost-effective manner. It involves creating a miniature model of the kitchen on a table and testing out different layouts, appliances, and color schemes.
The kitchen is often considered the heart of the home, where families gather to cook, eat, and share stories. As such, it is crucial to design a kitchen that is not only functional but also visually appealing. This is where a kitchen table titration lab comes in.
Kitchen table titration lab
is a process where homeowners can experiment with different design elements and materials for their kitchen in a controlled and cost-effective manner. It involves creating a miniature model of the kitchen on a table and testing out different layouts, appliances, and color schemes.
Cost-Effective and Time-Saving Solution
 A kitchen table titration lab is a cost-effective and time-saving solution for homeowners who are looking to redesign their kitchen. Instead of spending thousands of dollars on materials and labor for a full-scale kitchen renovation, homeowners can test out different design options on a smaller scale. This allows them to make informed decisions about the final design without breaking the bank.
A kitchen table titration lab is a cost-effective and time-saving solution for homeowners who are looking to redesign their kitchen. Instead of spending thousands of dollars on materials and labor for a full-scale kitchen renovation, homeowners can test out different design options on a smaller scale. This allows them to make informed decisions about the final design without breaking the bank.
Customize Your Kitchen to Your Needs
 Every family has different needs and preferences when it comes to their kitchen. Some may need more counter space, while others may prioritize storage. With a kitchen table titration lab, homeowners can customize their kitchen according to their specific needs and lifestyle. They can experiment with different layouts and configurations to find the perfect fit for their family.
Every family has different needs and preferences when it comes to their kitchen. Some may need more counter space, while others may prioritize storage. With a kitchen table titration lab, homeowners can customize their kitchen according to their specific needs and lifestyle. They can experiment with different layouts and configurations to find the perfect fit for their family.
Design with Confidence
 Designing a kitchen can be a daunting task, especially for those who are not familiar with design principles. A kitchen table titration lab provides homeowners with the opportunity to experiment and play around with different design elements without the pressure of making a permanent decision. This allows them to gain confidence in their design choices and create a kitchen that they can be proud of.
In conclusion, a kitchen table titration lab is an essential tool in the
house design
process. It allows homeowners to create a functional and aesthetically pleasing kitchen that is customized to their needs and preferences. With its cost-effective, time-saving, and confidence-building benefits, a kitchen table titration lab is a must-have for any homeowner looking to design their dream kitchen.
Designing a kitchen can be a daunting task, especially for those who are not familiar with design principles. A kitchen table titration lab provides homeowners with the opportunity to experiment and play around with different design elements without the pressure of making a permanent decision. This allows them to gain confidence in their design choices and create a kitchen that they can be proud of.
In conclusion, a kitchen table titration lab is an essential tool in the
house design
process. It allows homeowners to create a functional and aesthetically pleasing kitchen that is customized to their needs and preferences. With its cost-effective, time-saving, and confidence-building benefits, a kitchen table titration lab is a must-have for any homeowner looking to design their dream kitchen.














.PNG)