Sodium chloride, also known as NaCl, is the chemical compound that makes up the familiar pantry staple, table salt. This ubiquitous seasoning is a mineral that can be found in every household and is used in almost every dish. But what makes sodium chloride so special? Let's dive into the world of this essential compound and discover its properties and uses.Sodium chloride: The Most Common Kitchen Table Condiment
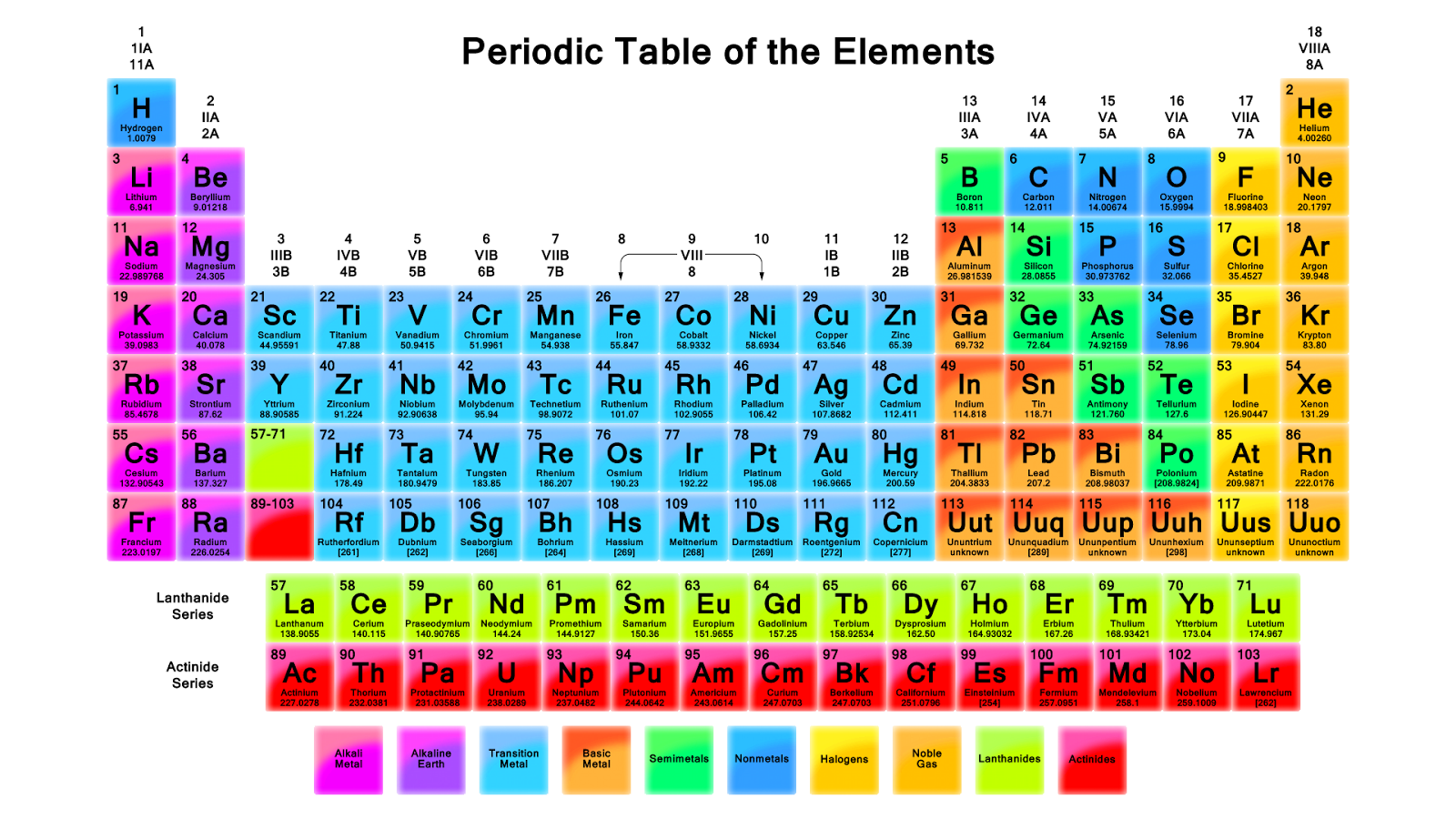
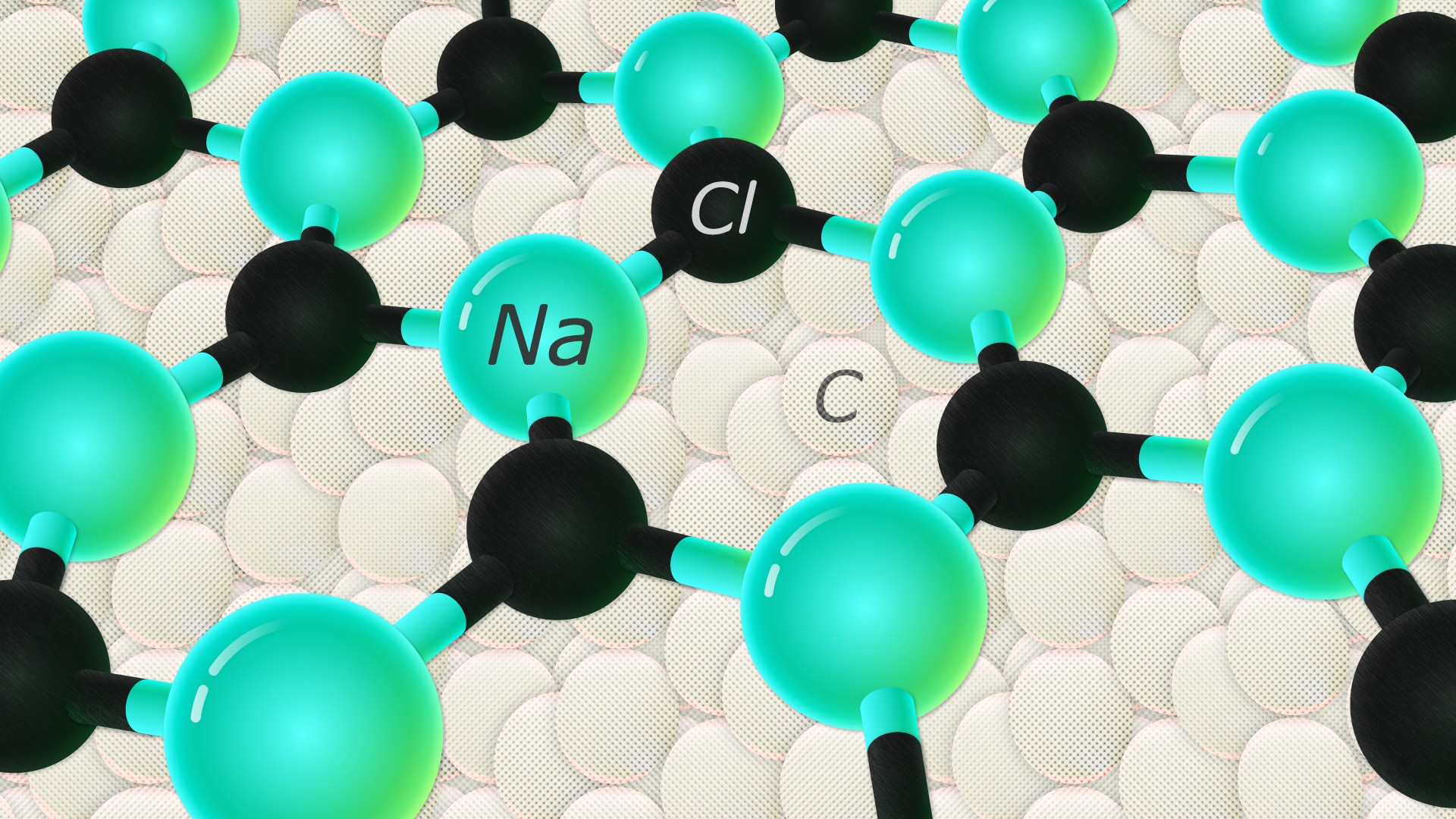
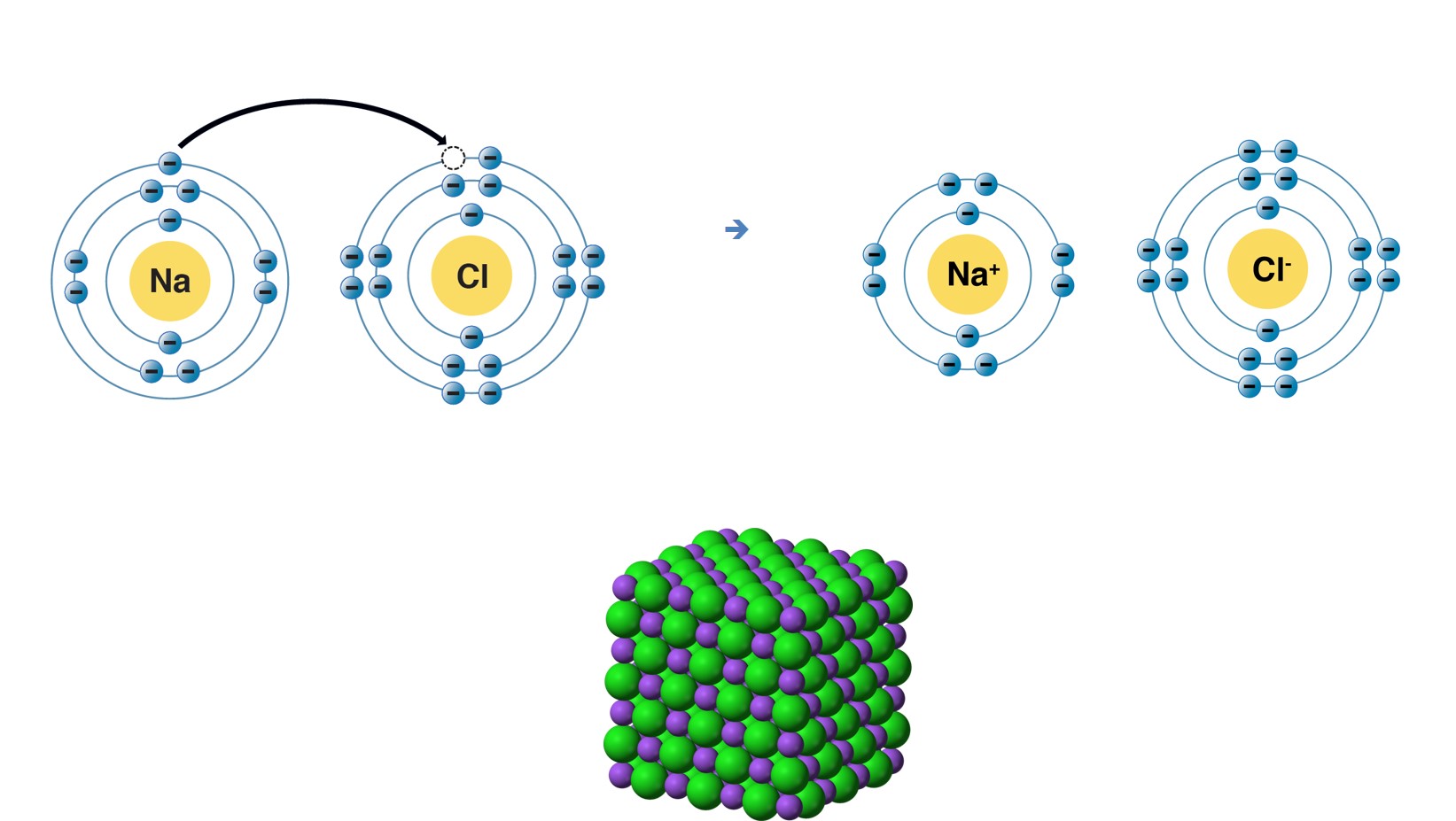
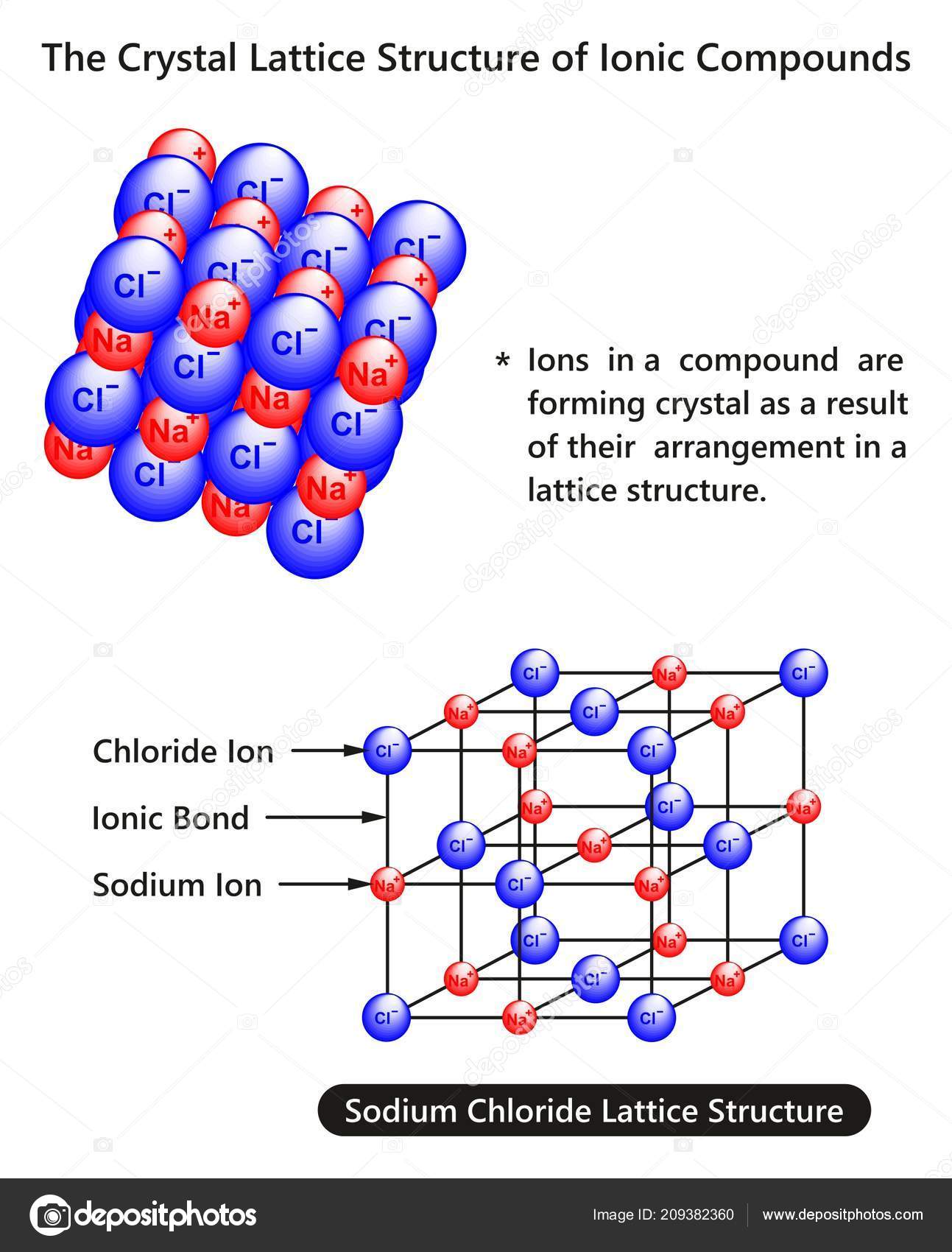
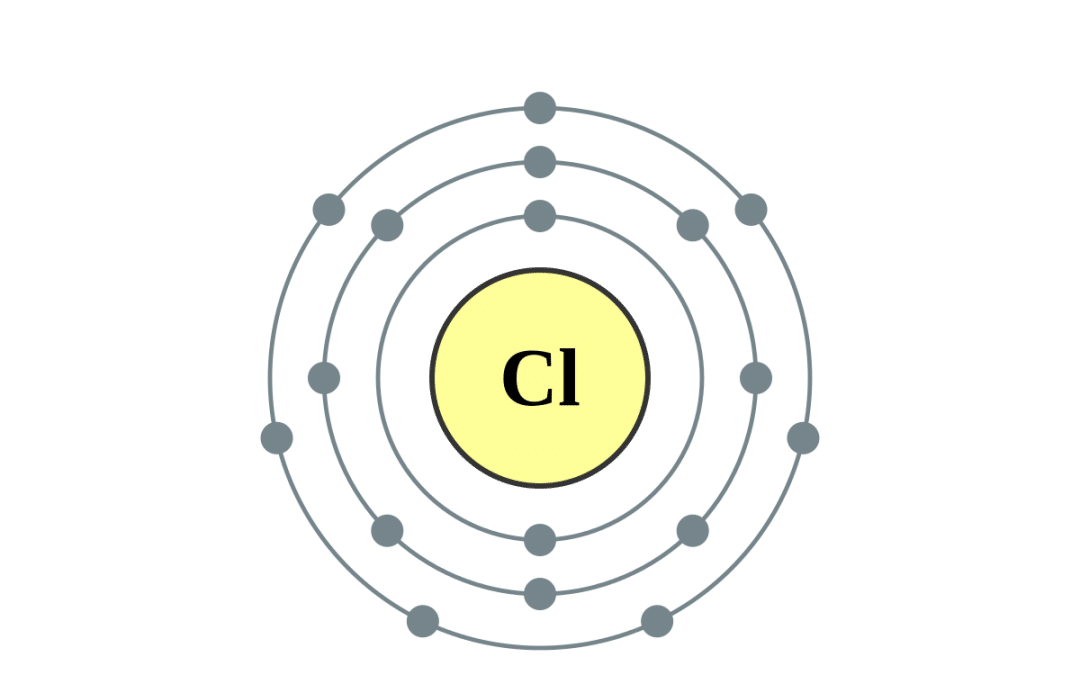
Sodium chloride is an ionic compound, which means it is made up of positively and negatively charged ions. In the case of sodium chloride, these ions are sodium (Na+) and chloride (Cl-). It is a naturally occurring mineral that is found in large deposits underground, and it can also be produced synthetically in laboratories.What is Sodium Chloride?
Table salt is the most common form of sodium chloride and is used in cooking, baking, and as a seasoning. It is typically made up of 97-99% sodium chloride, with the remaining percentage composed of trace minerals such as calcium, potassium, and magnesium. These trace minerals give table salt its characteristic flavor and color.Table Salt: The Most Common Form of Sodium Chloride
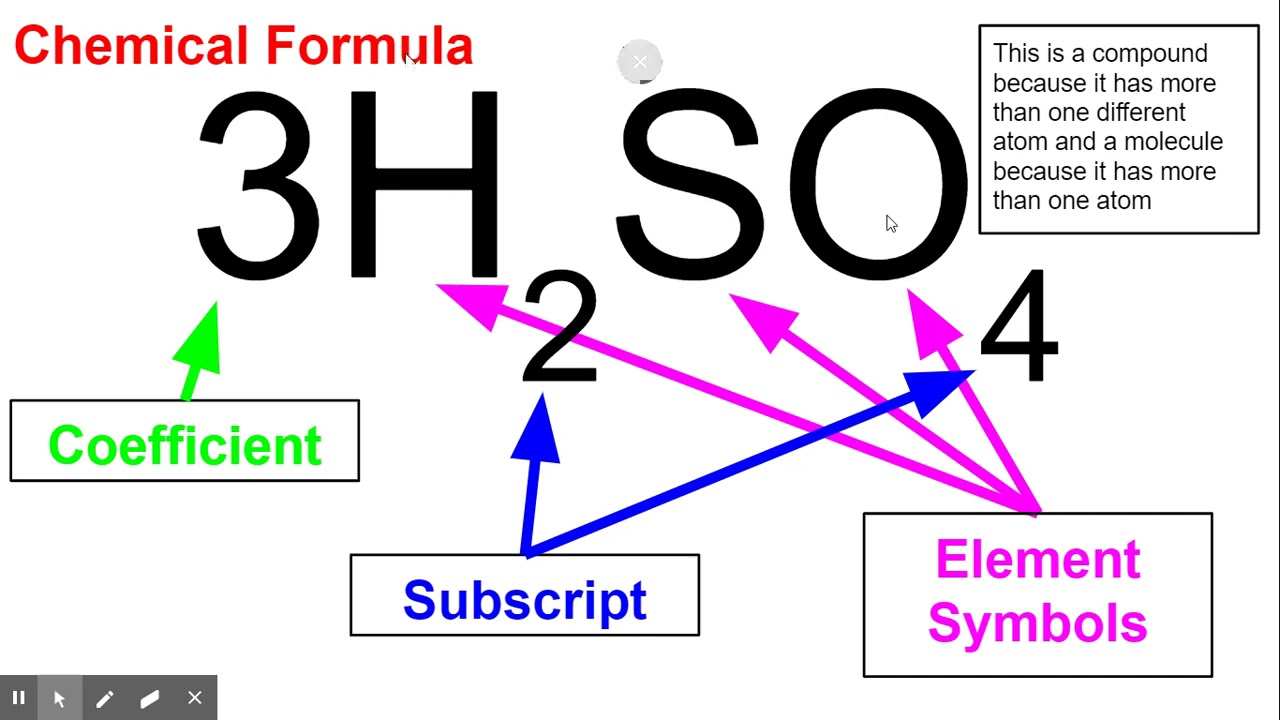
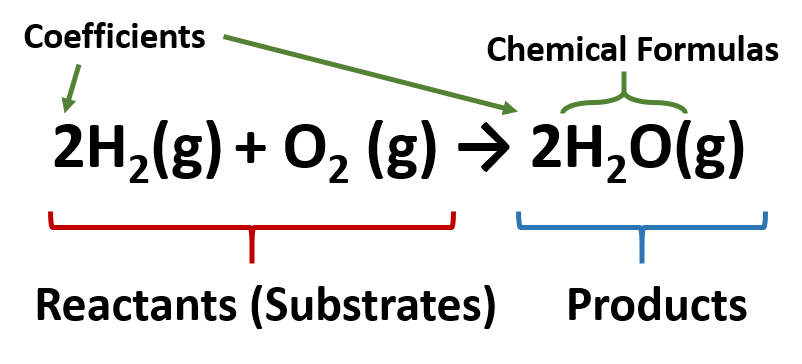
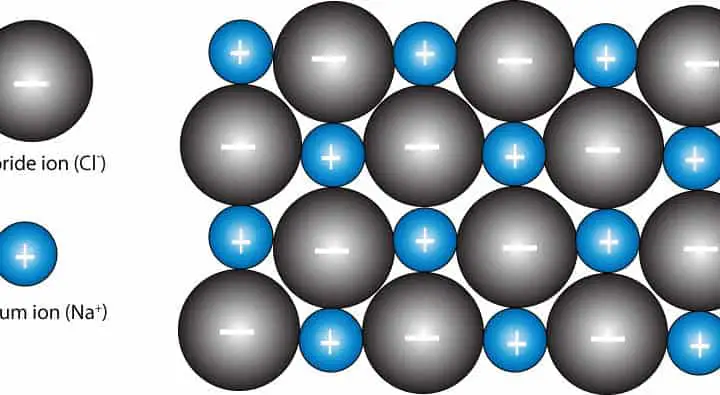
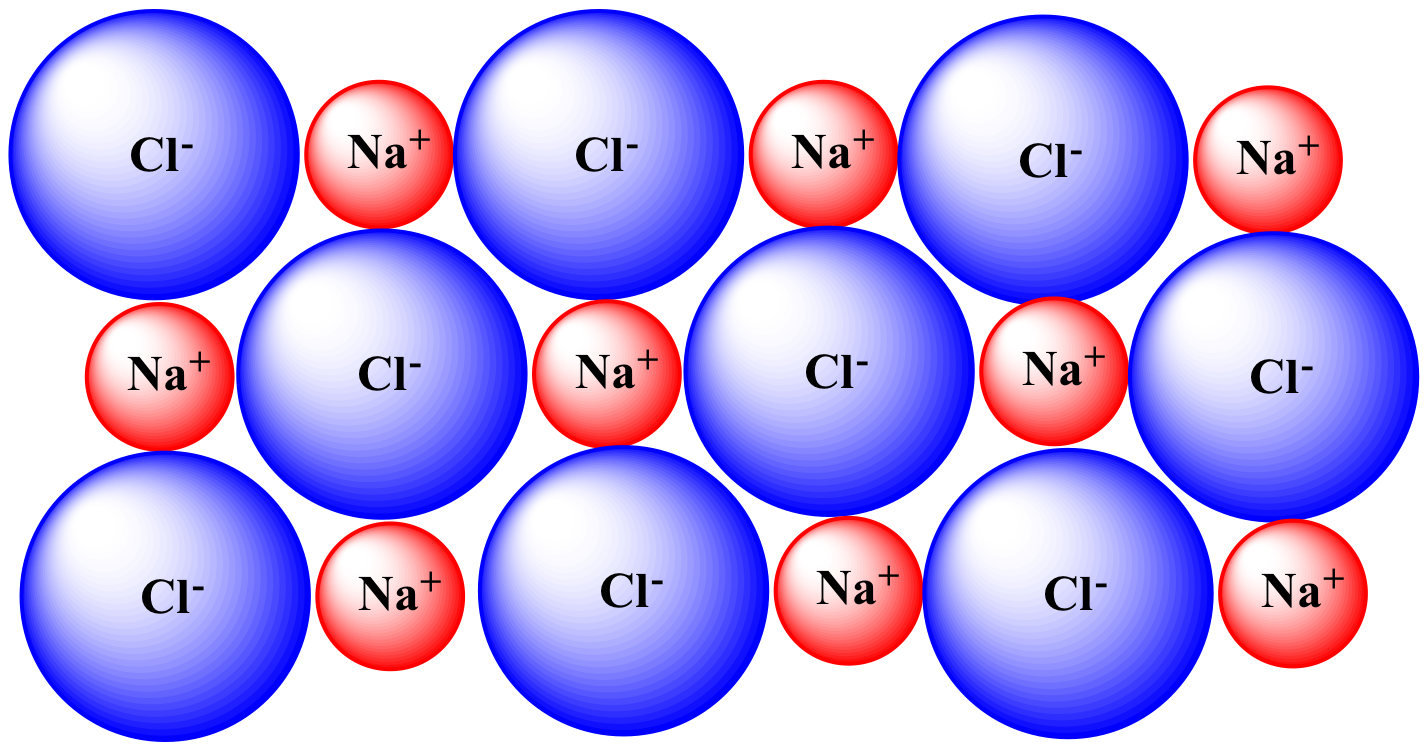
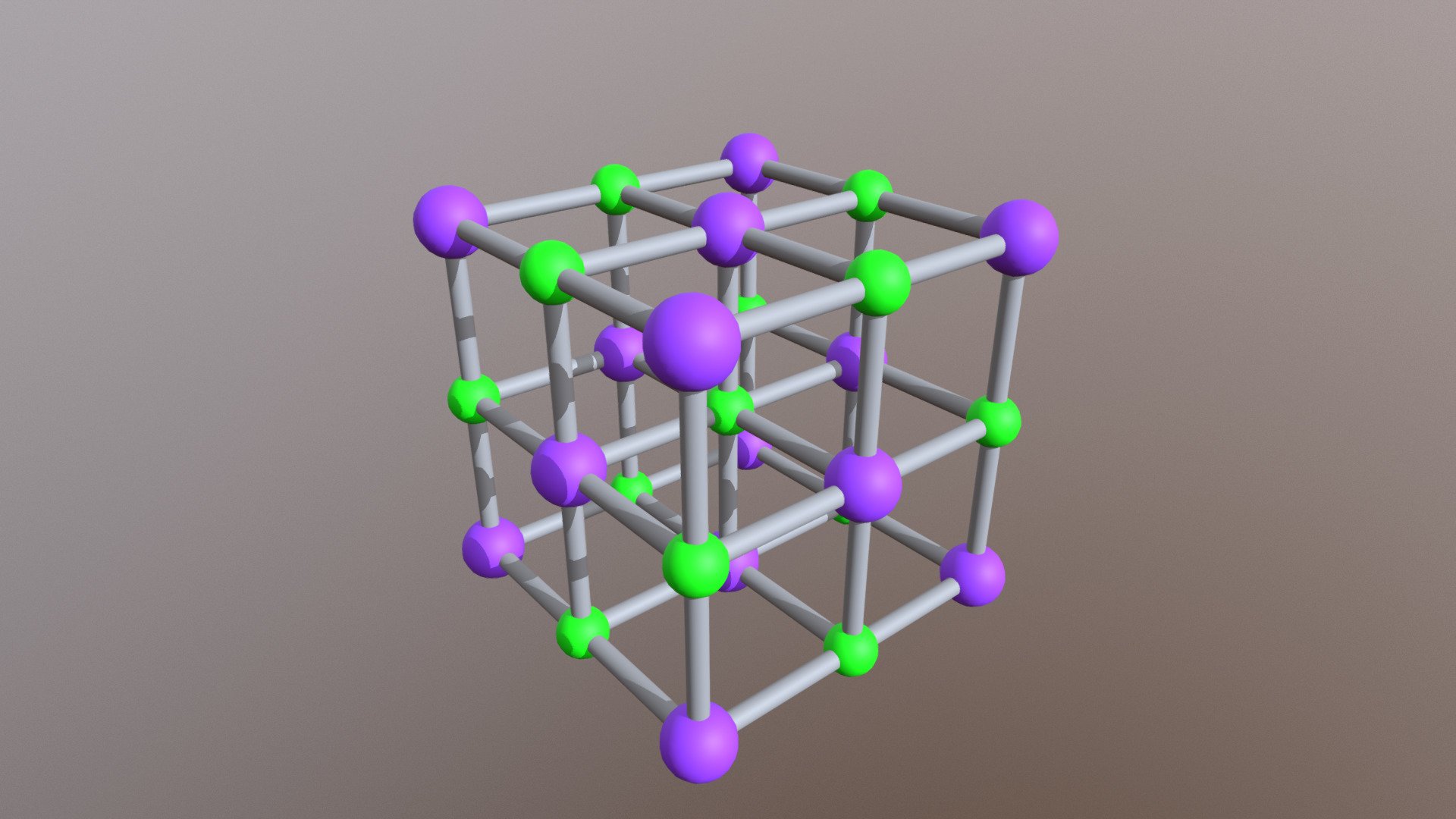
The chemical formula for sodium chloride is NaCl, which means it is made up of one sodium atom and one chlorine atom. When these two elements combine, they form a stable ionic compound with strong electrostatic forces that hold the ions together. This results in the formation of tiny, cube-shaped crystals, which give table salt its grainy texture.The Chemistry Behind Sodium Chloride
Both sodium and chlorine are essential for the human body to function properly. Sodium plays a vital role in maintaining fluid balance, nerve function, and muscle contraction. Chlorine, on the other hand, is necessary for the production of hydrochloric acid in the stomach and is also involved in the body's immune response.The Role of Sodium and Chlorine in the Body
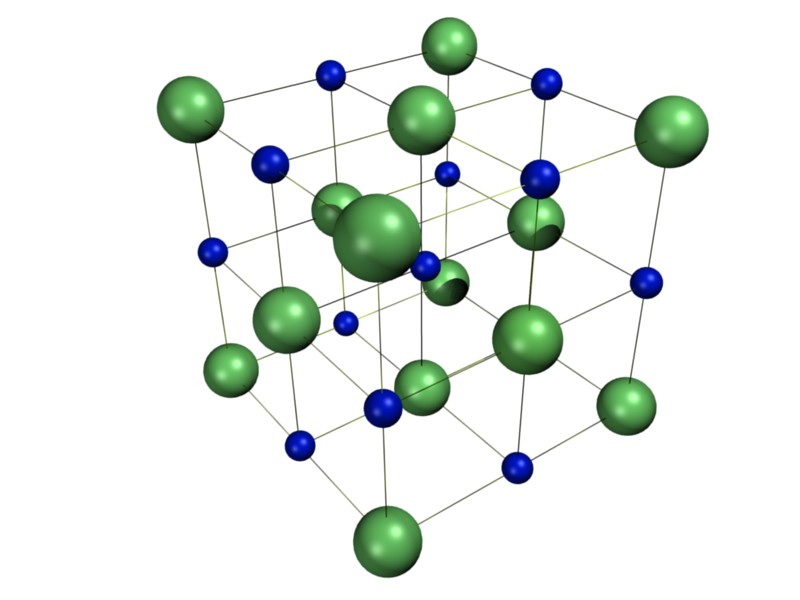
Sodium chloride has a face-centered cubic crystal structure, which means that each sodium ion is surrounded by six chloride ions, and vice versa. This arrangement gives sodium chloride its high melting and boiling points, making it stable at room temperature and ideal for use in cooking and food preservation.The Crystal Structure of Sodium Chloride
Aside from being a common table condiment, sodium chloride has many other uses. It is used in the production of chlorine gas, which is used in water treatment and the manufacturing of plastics and other chemicals. It also has industrial applications in the production of paper, soap, and glass.Uses of Sodium Chloride
While sodium chloride is an essential mineral, consuming too much of it can be harmful to our health. The recommended daily intake of sodium is no more than 2,300 milligrams, but the average American consumes more than 3,400 milligrams per day. This can lead to high blood pressure, heart disease, and other health issues.The Dangers of Consuming Too Much Sodium Chloride
Sodium chloride, also known as table salt, is a compound that is vital to our daily lives. It is a versatile and essential mineral that has many industrial and household uses. However, it is important to consume it in moderation and be mindful of our daily intake to maintain our health. So next time you reach for the salt shaker, remember the fascinating chemistry behind this common kitchen condiment.Conclusion
The Importance of Salt in Kitchen Design

Enhancing Flavor and Preserving Food
/85203934-56a12fcd5f9b58b7d0bce235.jpg) When we think of salt, the first thing that comes to mind is its role in enhancing the flavor of our food. But salt actually plays a much bigger role in kitchen design than just seasoning our meals. Salt is a natural preservative, which has been used for centuries to cure and preserve food. In fact, in the past, salt was so valuable that it was used as a form of currency. Adding salt to food not only enhances its taste, but it also helps to extend its shelf life, making it an essential ingredient in any kitchen.
When we think of salt, the first thing that comes to mind is its role in enhancing the flavor of our food. But salt actually plays a much bigger role in kitchen design than just seasoning our meals. Salt is a natural preservative, which has been used for centuries to cure and preserve food. In fact, in the past, salt was so valuable that it was used as a form of currency. Adding salt to food not only enhances its taste, but it also helps to extend its shelf life, making it an essential ingredient in any kitchen.
Creating Balance and Texture
 In addition to its preservative properties, salt is also important in creating balance and texture in dishes. It is a key ingredient in baking, helping to activate yeast and create a light and airy texture in breads and pastries. In savory dishes, salt helps to balance out flavors, bringing out the sweetness in vegetables and meats. It can also be used to add texture to dishes, such as in salt-crusted fish or salted caramel desserts.
In addition to its preservative properties, salt is also important in creating balance and texture in dishes. It is a key ingredient in baking, helping to activate yeast and create a light and airy texture in breads and pastries. In savory dishes, salt helps to balance out flavors, bringing out the sweetness in vegetables and meats. It can also be used to add texture to dishes, such as in salt-crusted fish or salted caramel desserts.
A Design Element in Itself
 Salt is not only an important ingredient in cooking, but it can also be a design element in itself. In modern kitchen designs, salt is often stored in stylish salt cellars or grinders, adding a touch of elegance to the kitchen. It can also be used as a finishing touch in plating, adding a pop of color and texture to dishes. The use of different types of salt, such as Himalayan pink salt or sea salt flakes, can also add an element of visual interest to a dish.
Salt is not only an important ingredient in cooking, but it can also be a design element in itself. In modern kitchen designs, salt is often stored in stylish salt cellars or grinders, adding a touch of elegance to the kitchen. It can also be used as a finishing touch in plating, adding a pop of color and texture to dishes. The use of different types of salt, such as Himalayan pink salt or sea salt flakes, can also add an element of visual interest to a dish.
Conclusion
 In conclusion, salt is not just a simple kitchen condiment, but an essential ingredient in cooking and kitchen design. Its ability to enhance flavor, preserve food, and create balance and texture make it a staple in every kitchen. So next time you reach for the salt shaker, remember the important role it plays in creating delicious and visually appealing dishes.
In conclusion, salt is not just a simple kitchen condiment, but an essential ingredient in cooking and kitchen design. Its ability to enhance flavor, preserve food, and create balance and texture make it a staple in every kitchen. So next time you reach for the salt shaker, remember the important role it plays in creating delicious and visually appealing dishes.
























/close-up-of-salt-shaker-spilled-on-table-953197320-5c3d4df6c9e77c0001eaf24f.jpg)











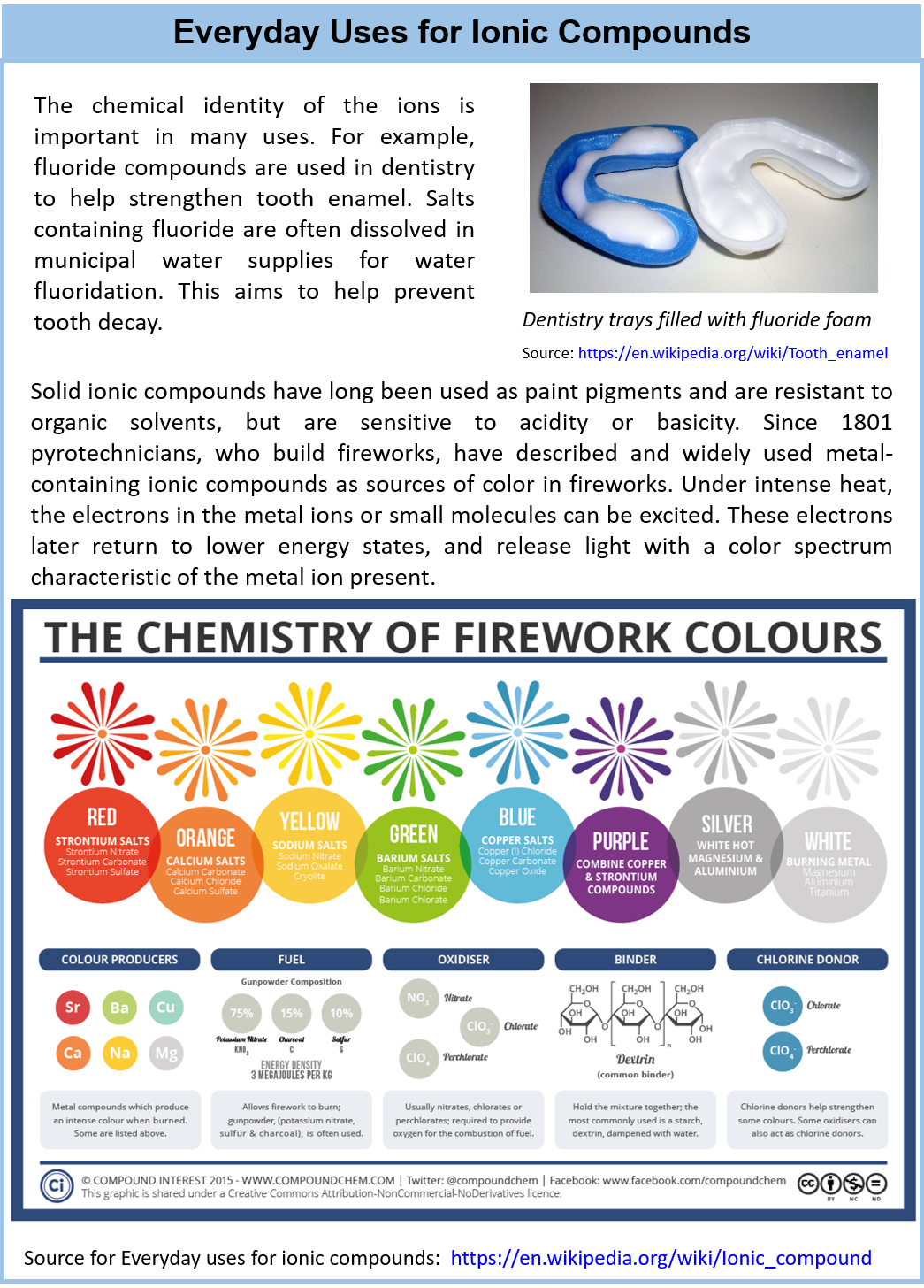


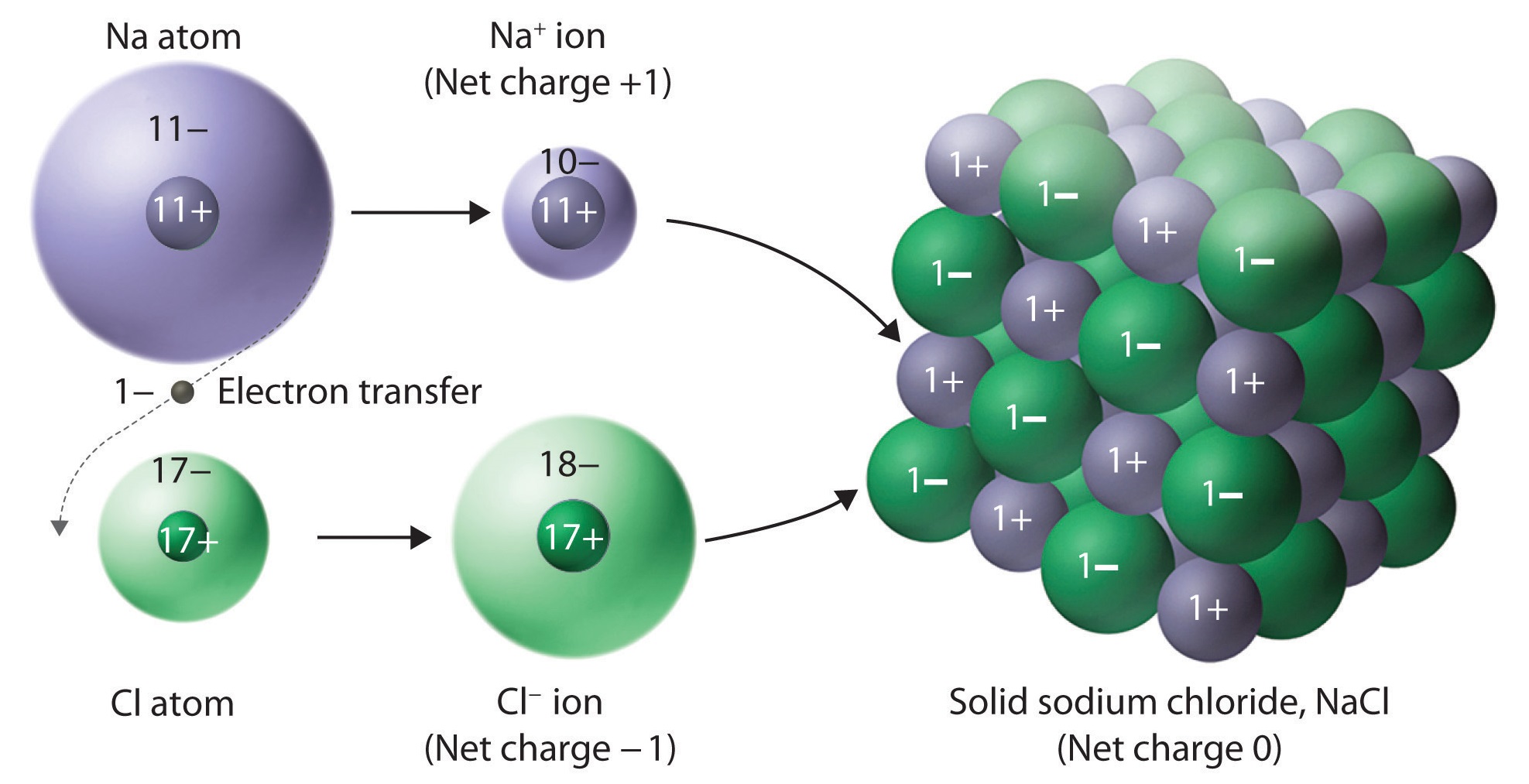
/ionic-bond-58fd4ea73df78ca1590682ad.jpg)
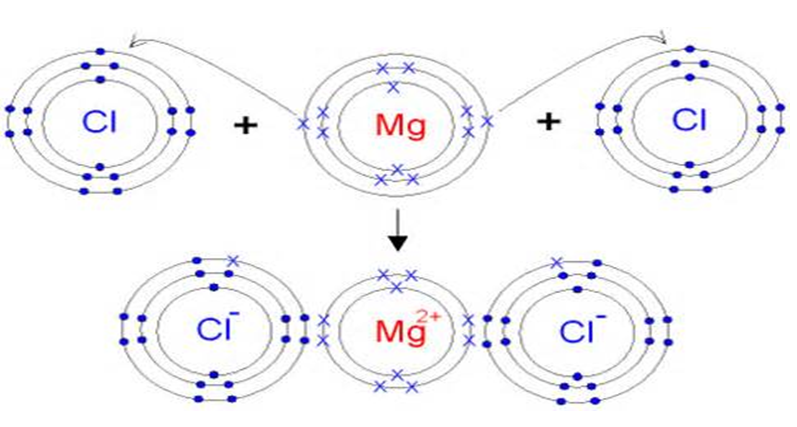




















/GettyImages-sb10067655by-001-1e3eb6ce621f4747b863b631b5cad181.jpg)
.jpg)










:max_bytes(150000):strip_icc()/chlorine-chemical-element-186451006-5810f6dd5f9b58564c68f382.jpg)






/chemistry-484263575-5aa68e43ff1b780036d9295d.jpg)